Lithium chemistries
The phrase 'lithium battery' is bandied about a lot on the internet lately and it would be easy to assume that all lithium batteries are the same, but they most definitely are not.
There are many different lithium battery technologies, each with different mix ratios of different chemistries, different energy to weight ratios and different levels of safety and expense. It's those different levels of safety in particular that warrant some discussion on this site.
Lithium ion or lithium iron?
- Lithium ion is a broad term for all lithium technology.
- Lithium iron is a specific lithium technology, also referred to as LiFePO4 or LFP or LYP (as in yttrium is in the mix) or just lithium ferrous.
Lithium batteries have received a lot of bad press because of the two homonyms ion and iron, so let's separate the broad term from the specific technology by calling the broad term for lithium technology lithium ion and the specific LiFePO4 technology lithium ferrous.
Lithium polymer
When people talk about lithium batteries exploding, they're talking about Lithium polymer batteries or LiPO. This is the chemistry that's used in batteries for model airplanes and phones. It has an excellent energy to weight ratio and batteries can be produced in lightweight casings to fit any shape required. However, they do have a tendency to catch fire or explode, especially when over charged or over discharged.
Lithium ferrous
Lithium iron phosphate (LiFePO4) and its close relative lithium yttrium iron phosphate (LiYFePO4) don't have the best energy to weight ratio, though it's still roughly twice as good as a lead acid battery's energy to weight ratio.
Lithium ferrous batteries do, however, have excellent stability, they don't explode and they don't self-combust. They make an excellent weight-saving, safe alternative to lead acid batteries in recreational vehicles and are also used in some electric vehicles, particularly those that are converted from carbon-based fuel to electric by home enthusiasts.
Lithium cobalt
Lithium cobalt is again a broad term for a number of lithium chemistries. This is the chemistry used in one form or another by manufacturers of electric vehicles. This particular lithium chemistry has one of the best energy to weight ratios available at present, but it is also one of the most dangerous chemistries. The battery management and cooling system required to keep these batteries safe is very complex and not something to be attempted by amateurs.
So if someone offers you some batteries from a used electric vehicle, check the technology. If it's a used production-line electric vehicle, stay away! There are YouTube videos of people who've got hold of used Tesla batteries and it's frightening. Unfortunately, these videos taint the lithium battery and electric vehicle industry with the one, poorly-researched brush.
An overview
Below are listed just some of the current lithium ion technologies used in batteries to give you an idea of the differences in efficiency and, most importantly, the difference in safety of these lithium technologies. Also highlighted is the specific technology of the cells that we use at T1 Lithium - LiYFePO4 - or lithium yttrium ferrous phosphate or simply LYP.
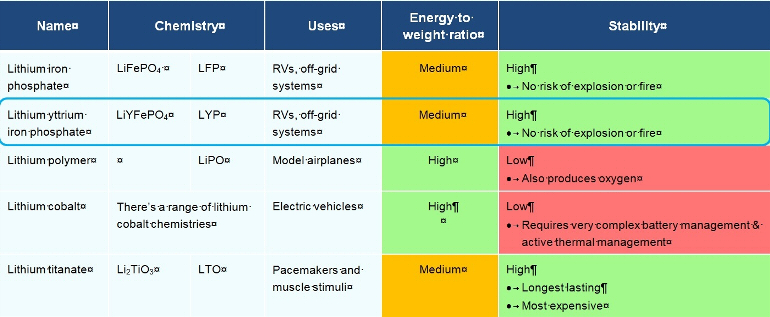
Both LiYFePO4 and LiFePO4 have only a medium energy to weight ratio, but have excellent stability, even if overcharged. Whilst weight is important in an RV, it's not nearly as important as in a model airplane. A model airplane may catch fire (hopefully in the air!), but you don't sleep in it, so the safety cost of ultra-low weight just isn't worth the risk. The trade-off in weight to get a stable battery - and let me say a battery more stable than one filled with sulphuric acid and water! - is only a trade-off in terms of other lithium batteries. You are still getting a much better energy to weight ratio than any lead acid battery.
A word about yttrium
In the Winston Thundersky cells that we use, yttrium is added to the LFP mix to improve the cycle life and lower the minimum temperature the cells can be charged or discharged at from 0.6°C for LFP to -30°C for LYP chemistry.
This can be done by changing the electrolyte mix in a LFP cell, but that also lowers the upper voltage range to below the temperatures than can be experienced in the Australian summer. The LYP compound is good for -30°C to 60°C without long term damage. The cells will still function outside these limits, but it would affect their cycle life.
So why go on about chemistry?
The reason for harping on about the different lithium technologies is not only to assure you that the cells we use are safe.
We also want to point out to our readers that it's important to understand the chemistry of the lithium batteries you buy, wherever or whomever you buy them from. For example, if you found someone selling some second-hand batteries from an electric car and you wanted to use these in your caravan or motorhome, you would be wise to make sure you're not buying lithium cobalt cells.
The chemistry of the batteries we use
At T1 Lithium, we use Thunder Sky WinstonTM cells from China and these use LiYFePO4 chemistry, or lithium ferrous yttrium iron phosphate.

A simple explanation of how lithium batteries work that explains their superior performance over lead acid batteries.
In order to know why lithium batteries work so well, it helps to understand how lead acid batteries work. The stark difference explains why lithium leads the way in charge and discharge speed, cycle life and longevity.